Phosgen Indikatorplakette Medic
Phosgen ist bei Normaltemperaturen gasförmig und gilt als sehr gesundheitsschädlich. Dieses Gas hat einen sehr schlechten Ruf, weil es im ersten Weltkrieg zusammen mit Chlor als Kampfgas eingesetzt wurde. Viele von uns haben schreckliche Bilder von schwer verätzten Soldaten im Kopf, wenn die Vokabel Phosgen erwähnt wird.
Heutzutage ist Phosgen ein wichtiger Grundstoff in der chemischen Industrie. Es ist nicht untertrieben zu behaupten, dass unser heutiger Lifestyle ohne Materialien zu deren Herstellung es einer Phosgenierung bedarf, nicht denkbar wäre. Stellvertretend seien hier nur erwähnt: Zahlreiche Medikamente, aber auch Kunststoffe wie Polyurethane (Schäume, Kleber, Farben, Isoliermaterialien, Polster) und Polycarbonate (CDs, mechanische Karosserieteile, Bedienelemente, Gehäuse)
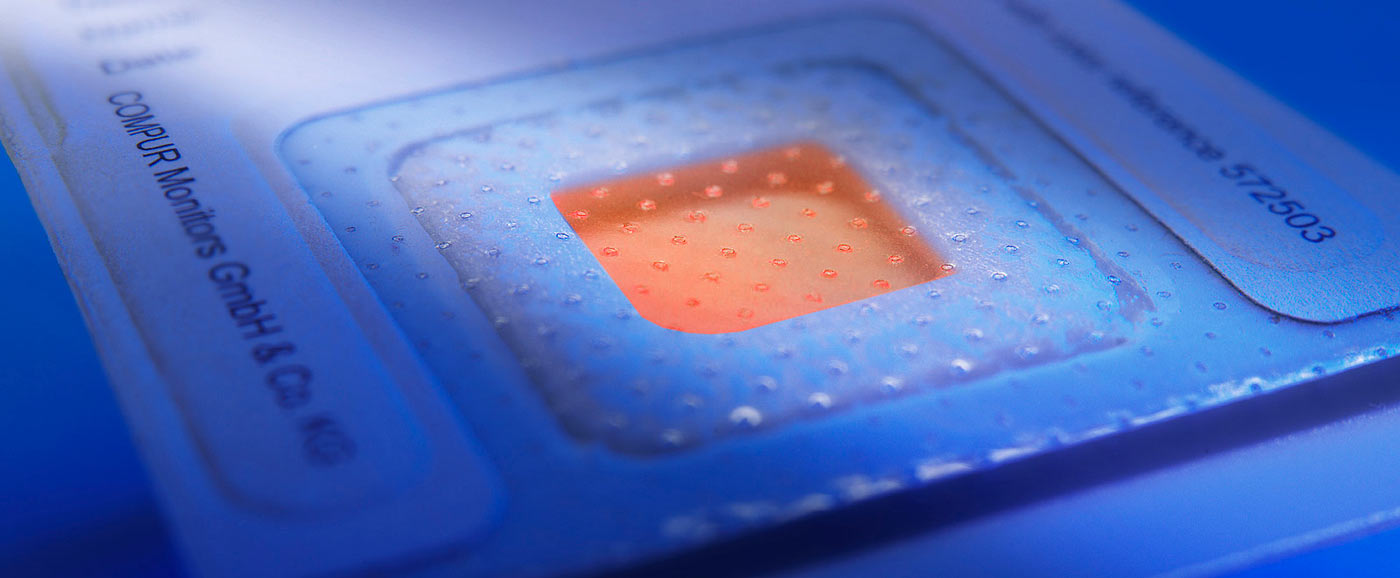
Selbstredend gelten bei der Herstellung und Verwendung von Phosgen allerhöchste Sicherheitsstandards. Phosgenanlagen werden rund um die Uhr von hochempfindlichen Messgeräten überwacht, so dass auch die allerkleinste Leckage entdeckt wird, bevor jemand zu Schaden kommen kann. Trotz all dieser Sicherheitsvorkehrungen kann niemand zur Gänze ausschließen, dass nicht doch einmal jemand exponiert wird. Selbst wenn dies der Fall sein sollte ist es noch kein Beinbruch. Eine Phosgenintoxikation ist therapierbar – vorausgesetzt, der behandelnde Arzt hat genaue Informationen über die aufgenommene Dosis.
Im Unterschied zu allen anderen toxischen Gasen erzeugt eine Phosgenintoxikation nämlich keine unmittelbaren Symptome. Deshalb besteht die Gefahr, dass ein Mitarbeiter, der eine Exposition erfahren hat nicht ausreichend behandelt oder übertherapiert wird. Der Arzt muss daher unbedingt die die gesamte aufgenommene Dosis, das ist das Produkt aus Konzentration * Zeit kennen, um sich für die richtige Therapie zu entscheiden. Die Compur Indikatorplakette MEDIC zeigt genau dieses an. Sie verfärbt sich rot, und zwar umso intensiver je höher die Gesamtexposition ist. Die Plakette wird mit einem Farbstandard verglichen auf dem man exakt die Gesamtdosis Konzentration * Zeit in der Einheit ppm * min ablesen kann. Mit diesem Werkzeug – versehen mit dem Namen des Benutzers – hat der Arzt das nötige Wissen an der Hand, um einem exponierten Mitarbeiter die optimale Behandlung zukommen zu lassen.
Schnellnavigation:
Technische Daten | Downloads
Technische Daten
| Messgas | COCl2 |
| Messbereich | 10 – 300 ppm * min |
| Format | 72 * 44 * 4 mm |
| Material | PET, PE, Papier |
| Farbwechsel | gelblich nach ziegelrot |
| Betriebstemperatur | -20°C bis +40°C |
| Feuchtebereich | rel. Feuchte 10 bis 100 % |
| Nutzungsdauer | 1 min bis 5 Tage ab Aktivierung |
| Lagertemperatur | +2°C bis 25°C |

Downloads
Achtung! Verwenden Sie ausschließlich den mitgelieferten Farbstandard. Vergleiche mit Darstellungen auf einem Bildschirm oder eigenen Drucken sind nicht zulässig. Bitte entsorgen Sie stets Ihre alten Standards, da Farben mit der Zeit verblassen können.

Dr. Josef von Stackelberg
Geschäftsführer COMPUR MONITORS GmbH & Co. KG
Kontaktieren Sie uns unverbindlich
Sie haben Fragen zu unseren Produkten oder wünschen eine unverbindliche Beratung? Wir freuen uns auf Ihre Kontaktaufnahme.


